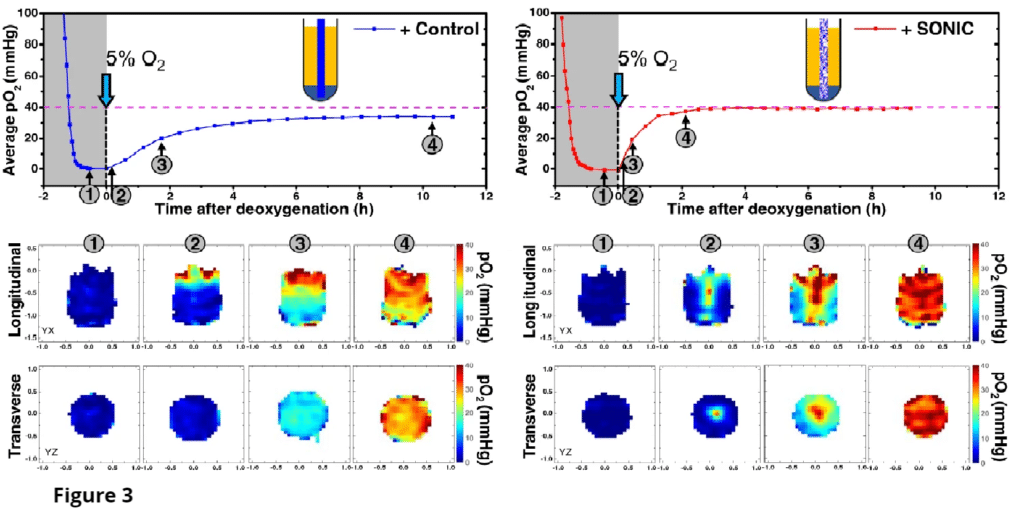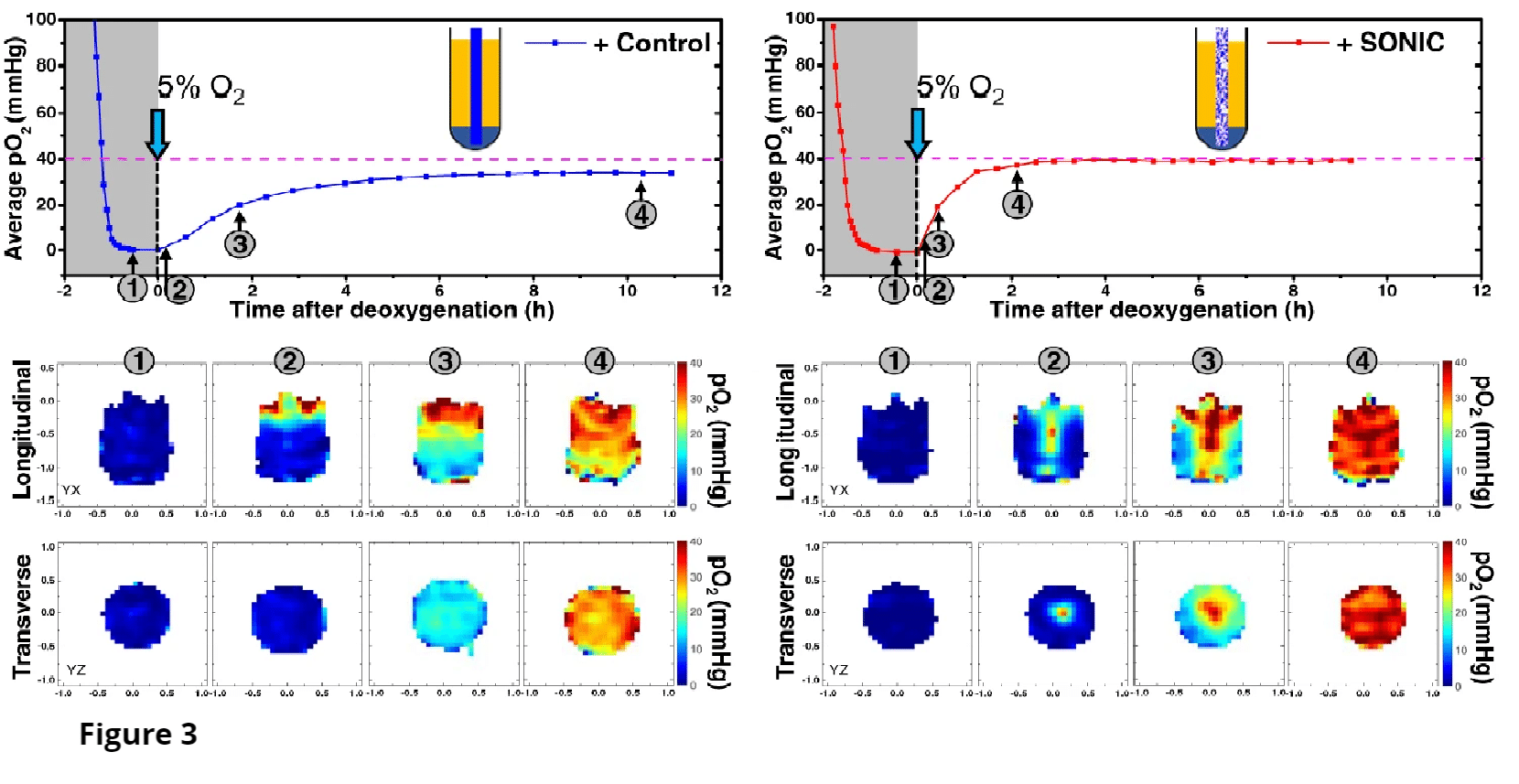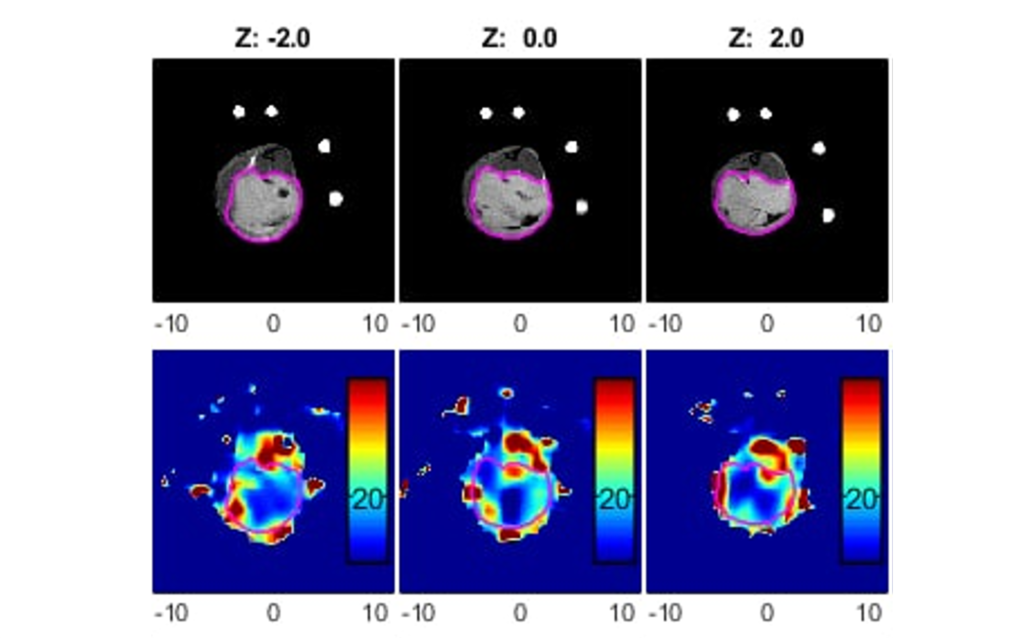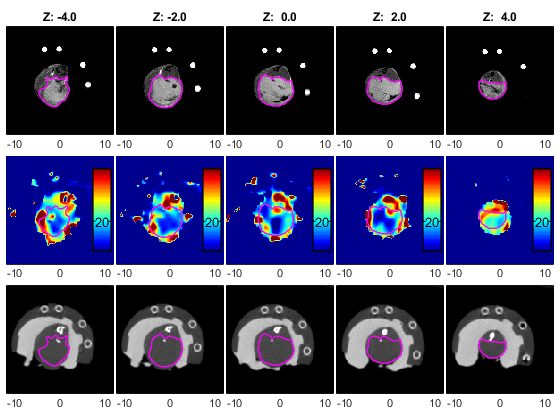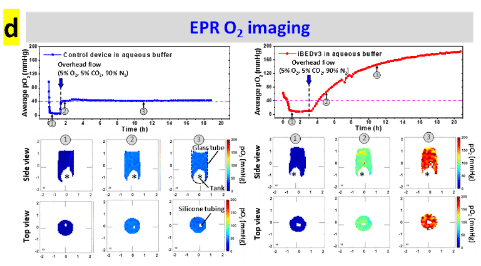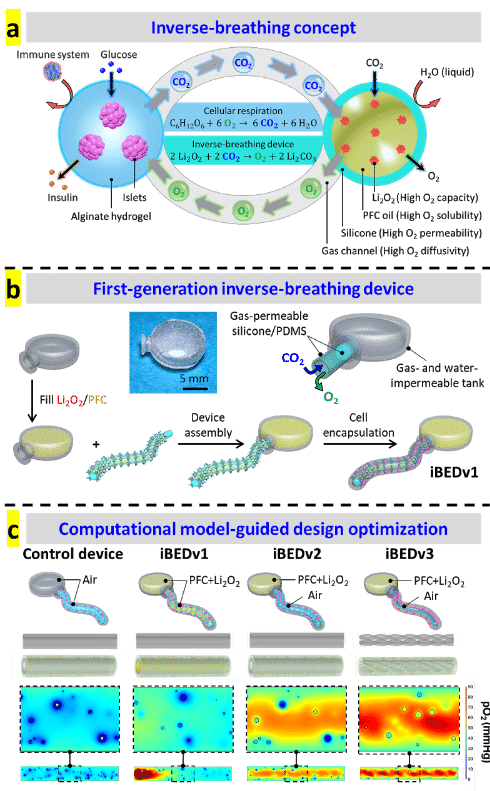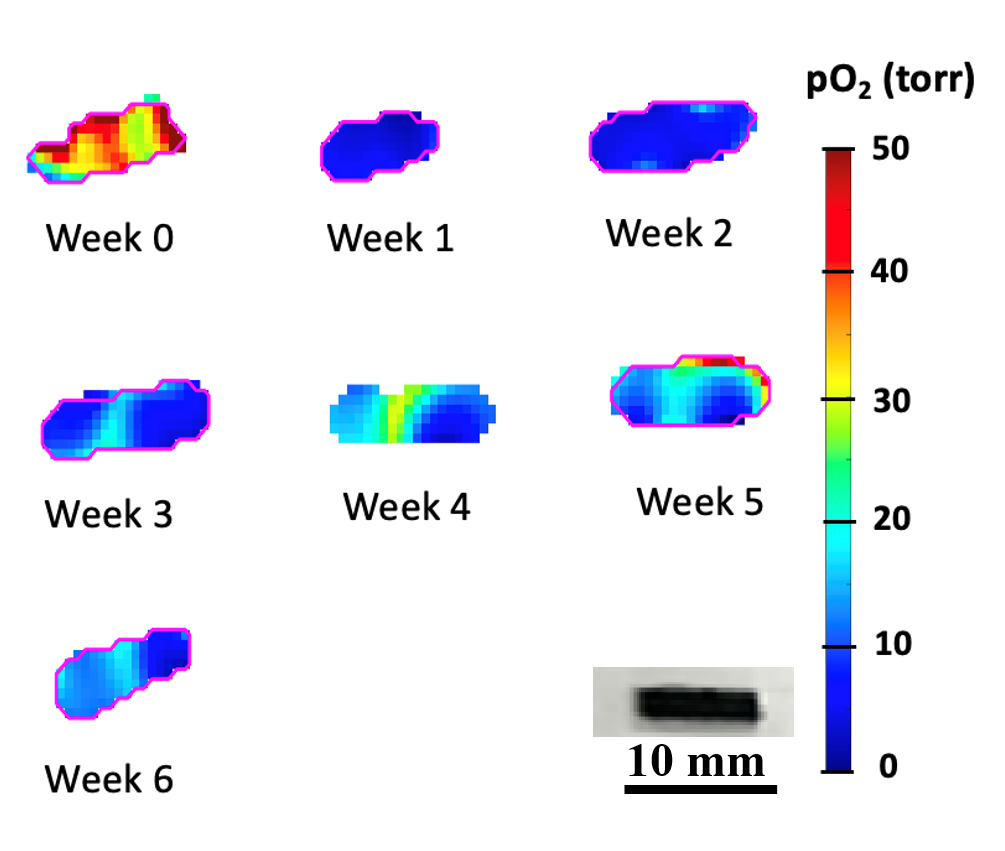
Oxygen sensitive LiPc probes implanted within the right dorsum subcutaneously, reporting oxygen tension over 6 weeks. Data obtained by JIVA-25™ oxygen imager.
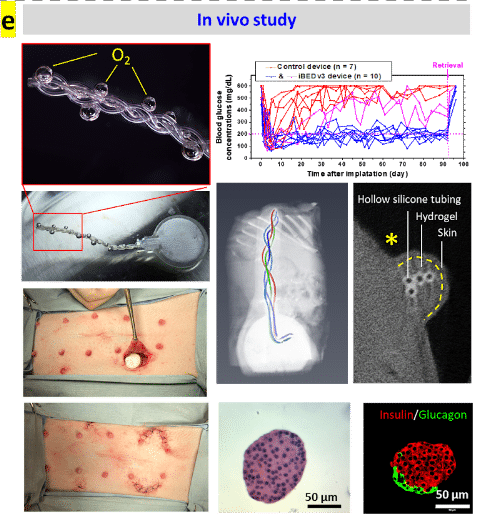
Tissue engineered grafts and cell encapsulation devices have significant potential to improve human health. The site of transplantation is a factor that has profound effect on graft survival. The ideal chosen site should be easy to access for implantation and retrieval, while providing adequate diffusion of nutrients and oxygen for support of cell survival until vascularization is established. The two most commonly used sites for implantation of tissue grafts and cell encapsulation devices are subcutaneous (SC) and intraperitoneal (IP). Evaluating the native oxygenation in these sites is valuable information towards understanding the physiological milieu of implants in vivo.
O2M’s recent peer-reviewed publication in Tissue Engineering Part C: Methods presents the first ever solid probe oxygen imaging, reporting pO2 values, of subcutaneous and intraperitoneal spaces in mice. We observe that solid probe oxygen imaging is a technology that offers researchers oxygenation data in vivo without needing further invasive measures or sacrifice. This study was funded by Juvenile Diabetes Research Foundation (JDRF) and will help scientists develop better therapies for type I diabetes as well as other health conditions.
Visit O2M’s Publication Page to learn about latest research in oxygen imaging.
O2M is at BioFAB and TERMIS!

Come see us in Manchester, New Hampshire!
Spring 2022 Meeting in the Millyard
June 7 – June 9
O2M will be presenting our latest research at the Poster Session
Wednesday June 8, Currier Museum, 5:30pm – 8:00pm
“In Vitro and In Vivo Oxygen Imaging of Islet Encapsulation Devices: Lessons Learned and Path Forward”

Come See Us in Toronto!
TERMIS-AM 2022
July 10 – July 13
Booth #107
Are you at TERMIS 2022? Make sure your plans include a visit to our booth in the Exhibition Hall. We hope to see you there!
O2M is Presenting at TERMIS!
Keynote Talk by Dr. Mrignayani Kotecha
“In Vitro and In Vivo Oxygen Imaging assessment of Islet Transplantation Devices”
Sessions 7, Non-invasive Imaging and Analysis of Engineered Tissues
Wednesday, July 13, 8:00 AM – 8:30 AM
Poster Presentation by Team O2M
“In Vivo Oxygen Imaging Of Oxygen Generating Cellular Implant Devices”
Poster Session 3
Tuesday, July 12, 4:30pm – 6:00pm
Upcoming O2M Webinar
 Register for Webinar
Register for Webinar
Moderator: Dr. Martyna Elas, Jagiellonian University, Krakow, Poland
About the Speaker:
Dr. Mark “Marty” Pagel has focused on molecular imaging research during the last 20 years in industry and academia. He is a Professor in the Departments of Cancer Systems Imaging and Imaging Physics at the University of Texas MD Anderson Cancer Center. In addition, Dr. Pagel has held leadership positions in professional societies, funding agencies, and scientific journals that focus on molecular imaging. Dr. Pagel’s current research focuses on CEST MRI, PET/MRI, photoacoustic imaging, and EPR imaging, for studies of tumor hypoxia, acidosis, vascular perfusion and enzyme activity, with mouse models of cancer and for clinical trials with cancer patients.
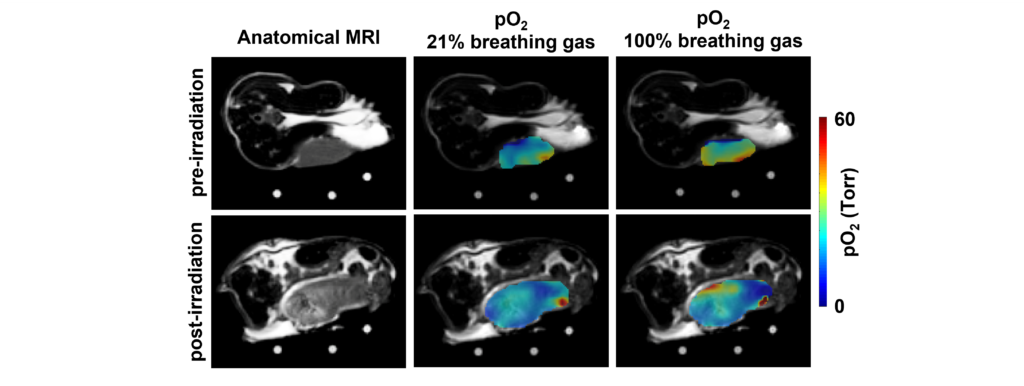
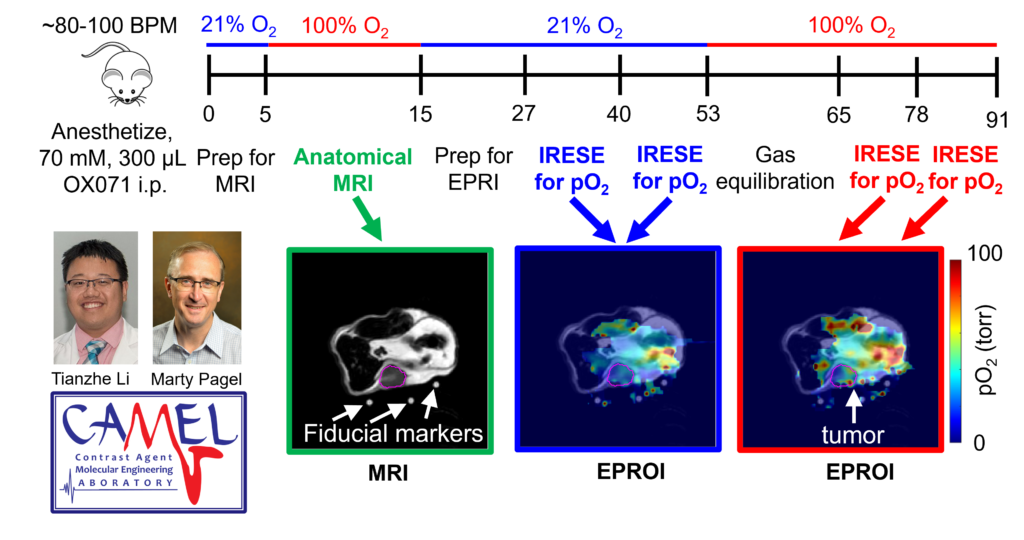
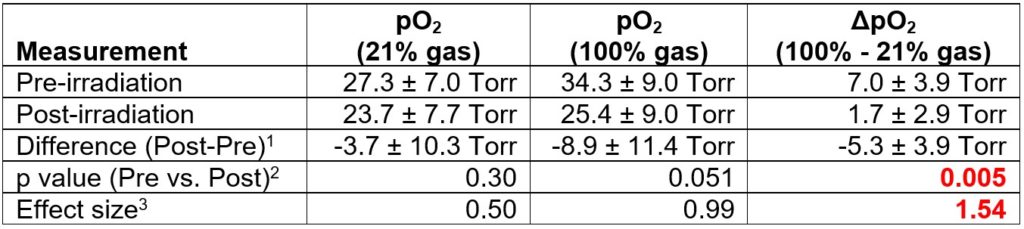
Abstract: Previous studies with EPR imaging have shown that measurements of tumor pO2 can indicate the status of hypoxia in the tumor microenvironment, which can be used to predict response to radiation therapy. To build on these previous studies, we are developing Oxygen Enhanced (OE) EPR imaging that challenges a pre-clinical tumor model with 21% O2 (medical grade air) and 100% O2 in the anesthetic carrier gas, which can be a useful biomarker for evaluating early response to radiation therapy. In addition, we are developing Dynamic Contrast Enhanced (DCE) EPR imaging that measures tumor vascular perfusion, as a complimentary biomarker for evaluating early response to cancer treatment. This presentation will also discuss our ongoing research studies that show how tumor hypoxia causes resistance to immunotherapy, and how reducing hypoxia can improve tumor control with immune checkpoint blockade.