Cell encapsulation represents a promising therapeutic strategy for many hormone-deficient diseases such as type I diabetes (T1D). However, adequate oxygenation of the encapsulated cells remains a challenge, especially in the poorly oxygenated subcutaneous site.
Recently, our collaborator, Dr. Minglin Ma's group at Cornell University, published a paper “An inverse-breathing encapsulation system for cell delivery” in Science Advances. This work reported a novel oxygen delivery system that generates oxygen (O2) for the islet cells from their own waste product, carbon dioxide (CO2), in a self-regulated (i.e. “inverse breathing”) way, supporting the long-term function of islets in the cell encapsulation device.
As lead author, Dr. Longhai Wang designed and fabricated the inverse-breathing device (iBED) by employing a gas-solid (CO2-lithium peroxide) reaction that was completely separated from the aqueous cellular environment by a gas-permeable membrane (Fig. a and b). Doctoral student Alexander Ernst performed computational modeling which guided device design optimizations (Fig. c).
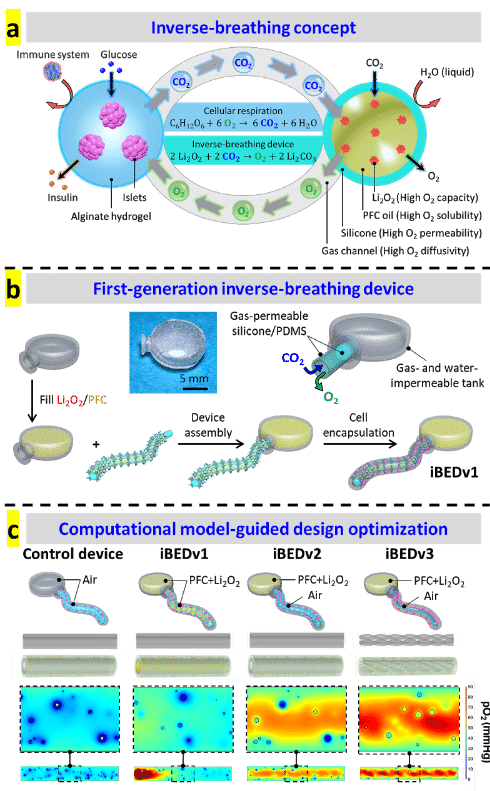
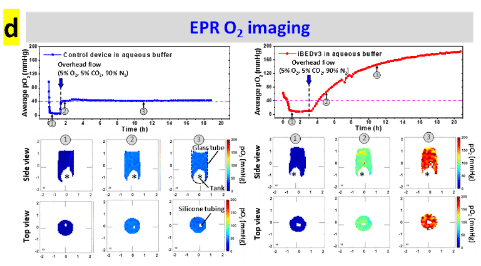
Oxygen mapping was performed using O2M's preclinical oxygen imager JIVA-25™ instrument at O2M's “Oxygen Measurement Core” facility to validate the CO2-regulated O2 release in the inverse-breathing devices (Fig. d).
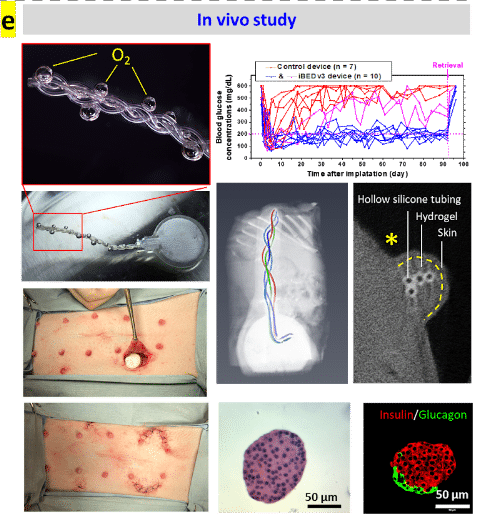
The iBED restored normoglycemia of immunocompetent diabetic mice for over 3 months. And functional islets were observed in scaled-up device implants in minipigs retrieved after 2 months (Fig. e). Furthermore, O2 supply may be extended indefinitely by the introduction of a tank replacement or formulation refilling modular design.
This inverse breathing device provides a potential system to support long-term cell function in the clinically attractive subcutaneous site by overcoming several outstanding challenges in oxygenating encapsulated cells and thus represents considerable progress in the use of translatable long-term O2-supplementing technologies for cell replacement therapies.
Read the complete article here: https://advances.sciencemag.org/content/7/20/eabd5835
