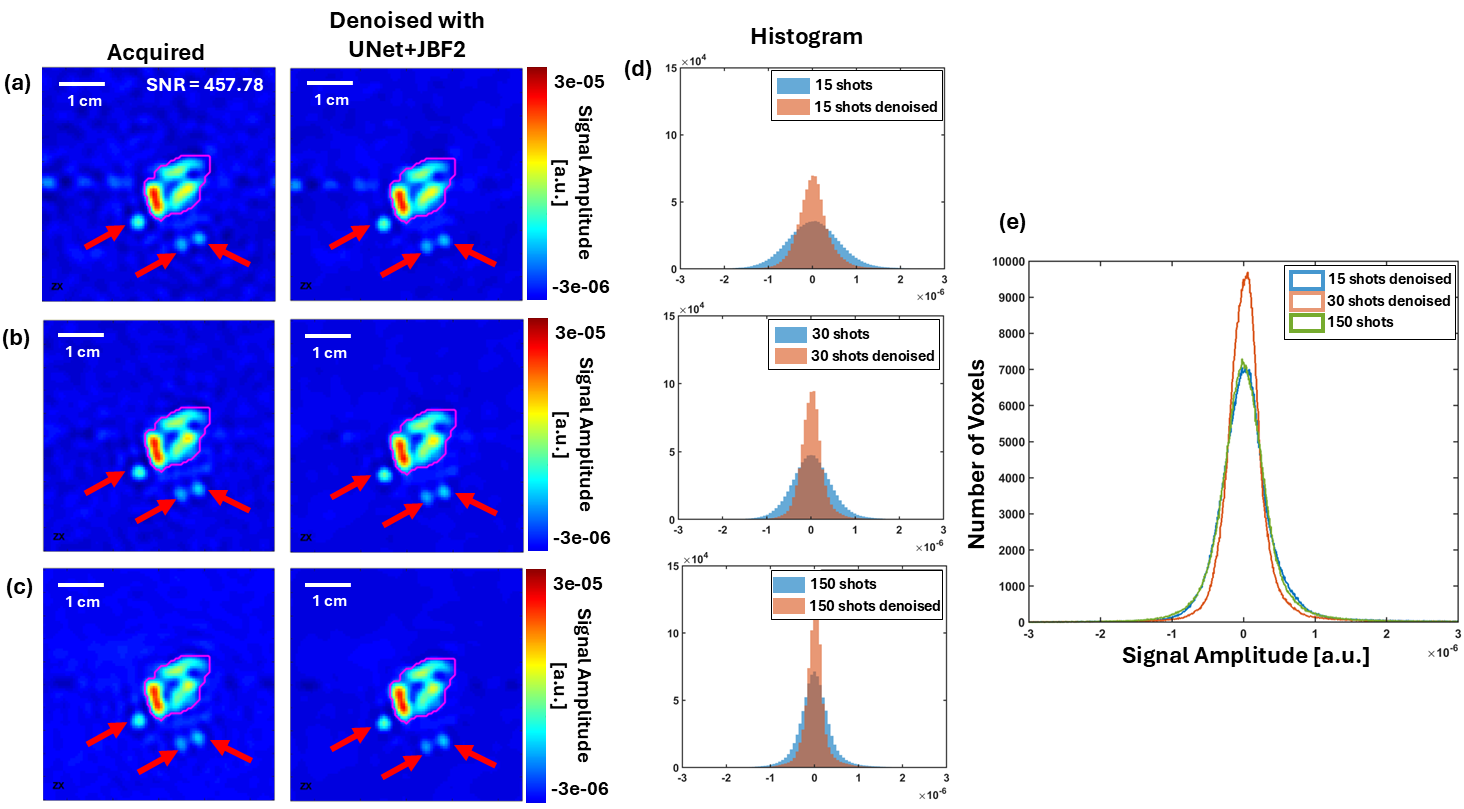
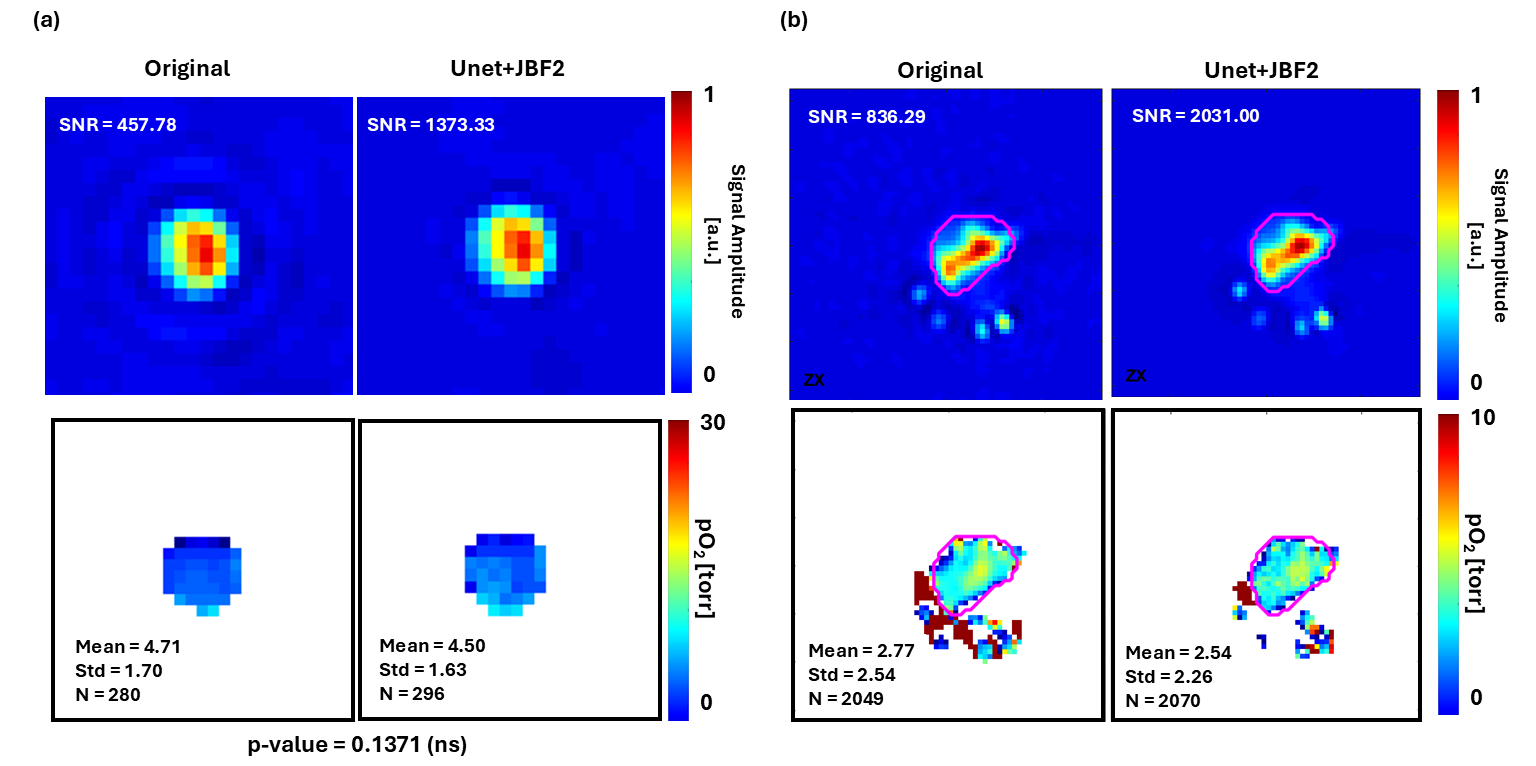
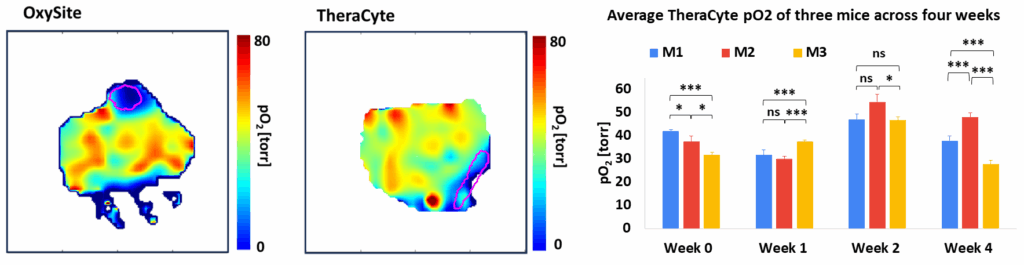
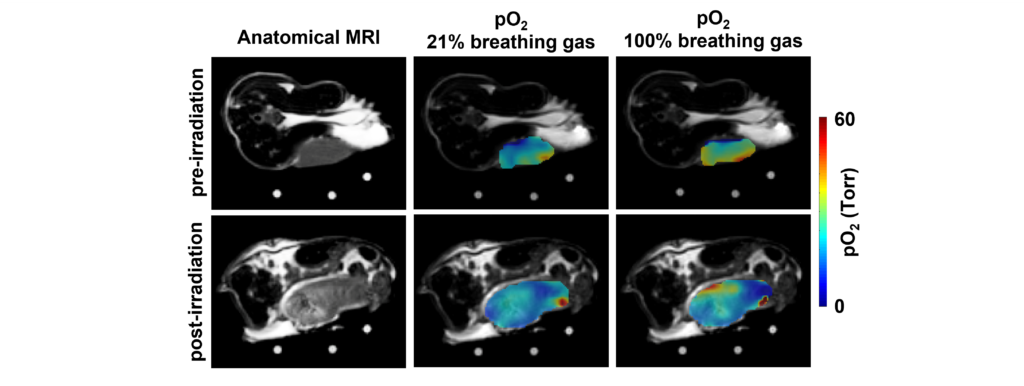
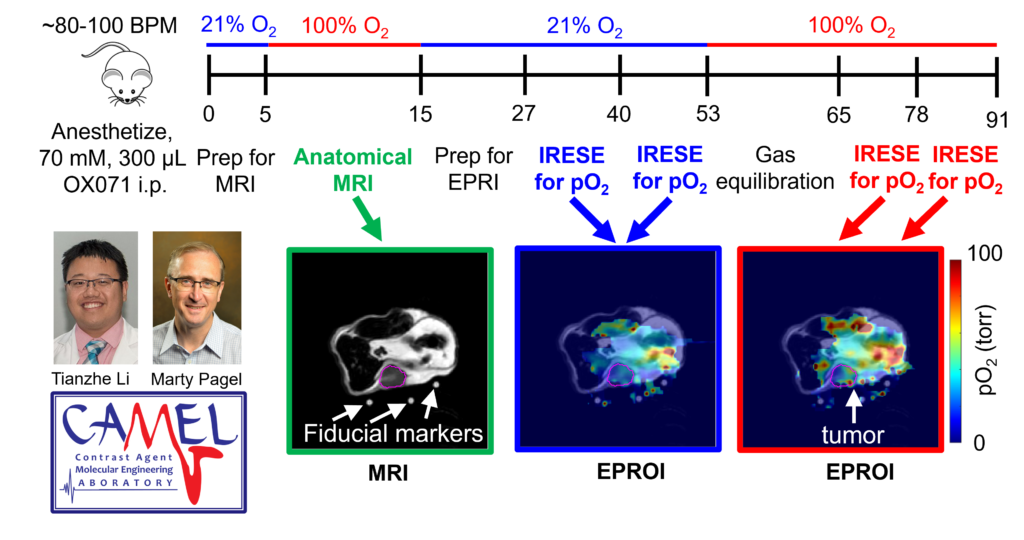
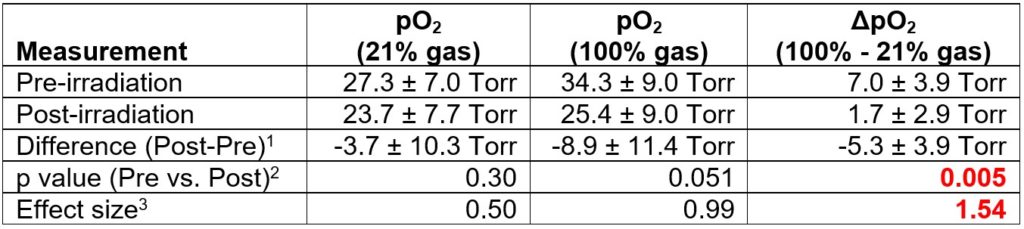
Oxygen Imaging of Cells for Viability Assessment
Cell viability is an essential measurement for cell therapy, tissue-engineered medical products (TEMPs), drug development, and many other biological processes and products. These systems rely on viable, healthy, and functional cells to work as intended.
However, current assay-based methods for assessing cells are destructive and inadequate. In our recently published paper in npj imaging, we report partial oxygen pressure (pO2) maps of cells in an environmentally controlled multi-well plate as a new method to obtain a 3D, longitudinal assessment of cells without destroying them in the process. The data presented here could be useful to every biological lab.
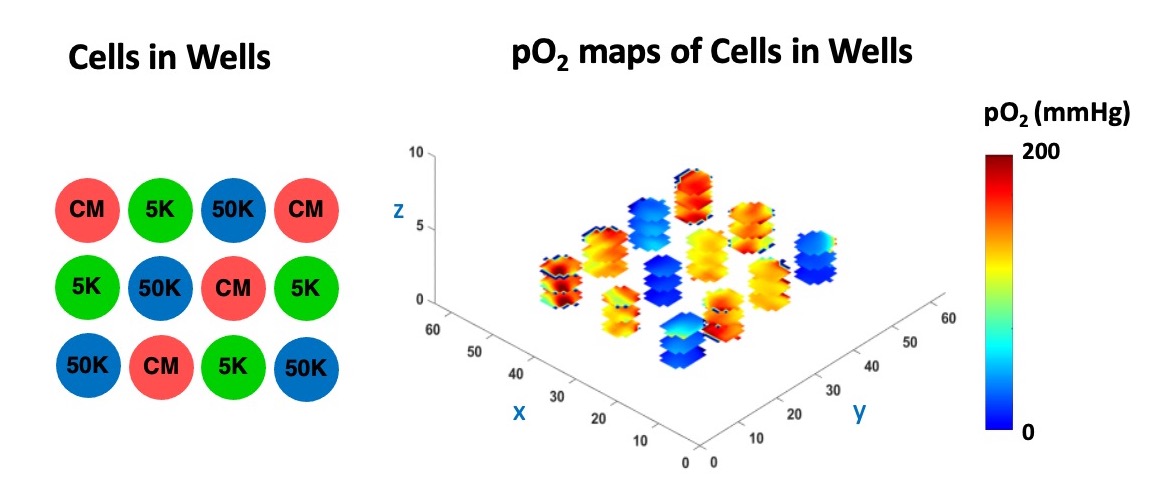
Using oxygen imaging of adherent and non-adherent cells seeded in an environment-controlled multi-well plate incubator-resonator, we demonstrated that:
- We can assess cells’ metabolic activities longitudinally and in 3D without destroying them in the process for up to 24 hours.
- We can distinguish between live and dead cells.
- The cells can be hypoxic even in the incubator.
- We can assess cells’ metabolic activity in a culture medium, but more importantly, when also seeded in a hydrogel.
- The oxygen concentration of wells with low cell density is high and decreases exponentially with increasing cell seeding density. However, it changes with time as cells become dormant, differentiate, propagate, or die. Therefore, cell density is an important factor to control when using them for cell therapy and tissue engineering.
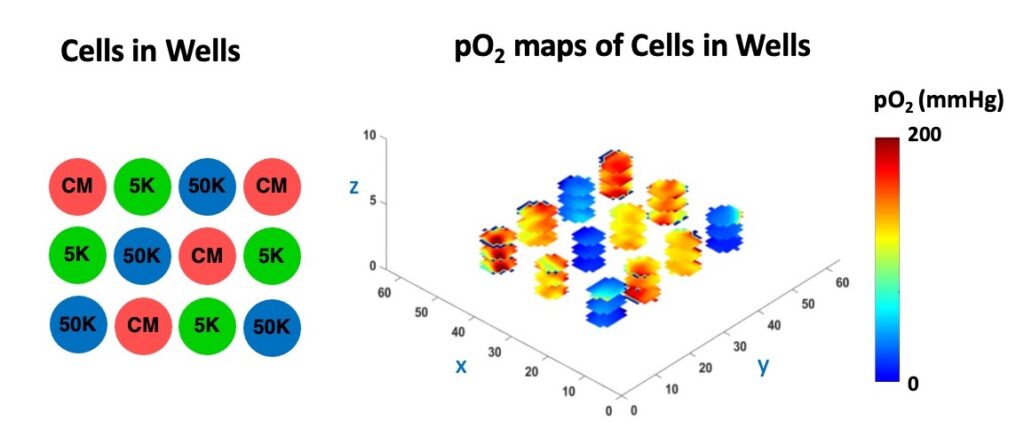
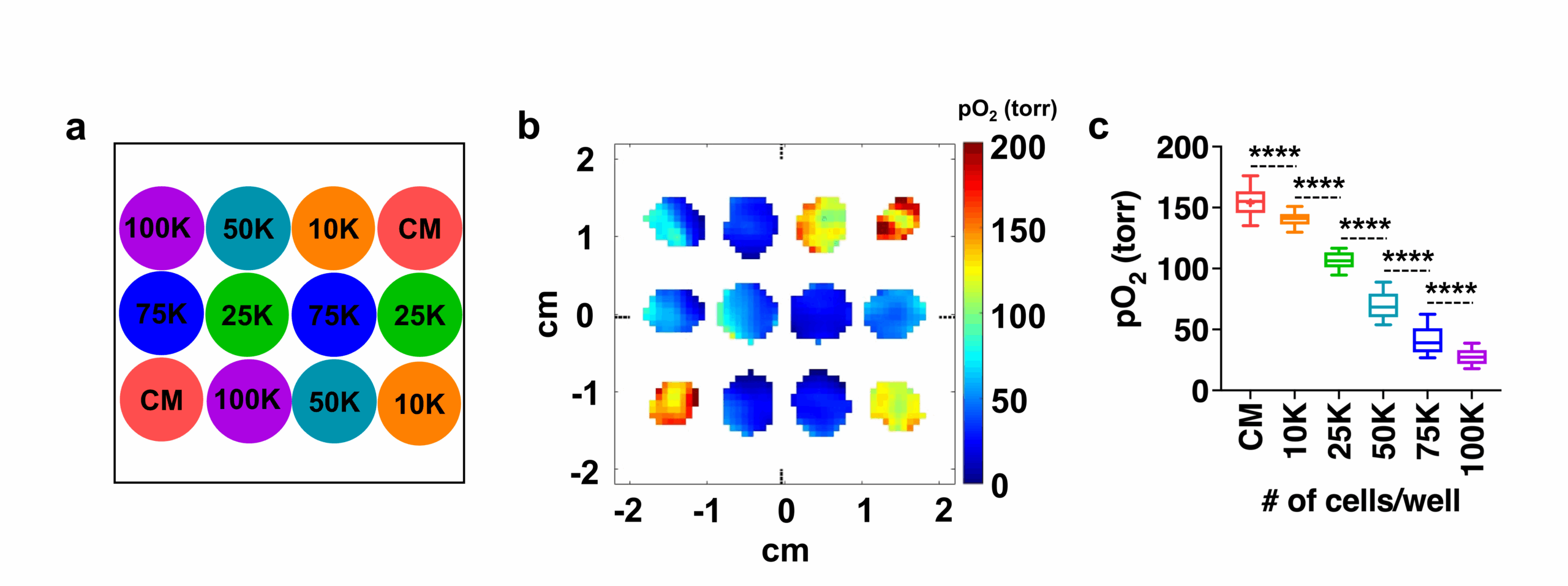
The figure below shows the pO2 maps and box plot of live HEK 293 cells seeded with different densities (low oxygen means the cells are alive and actively consuming oxygen, while dead cells do not).
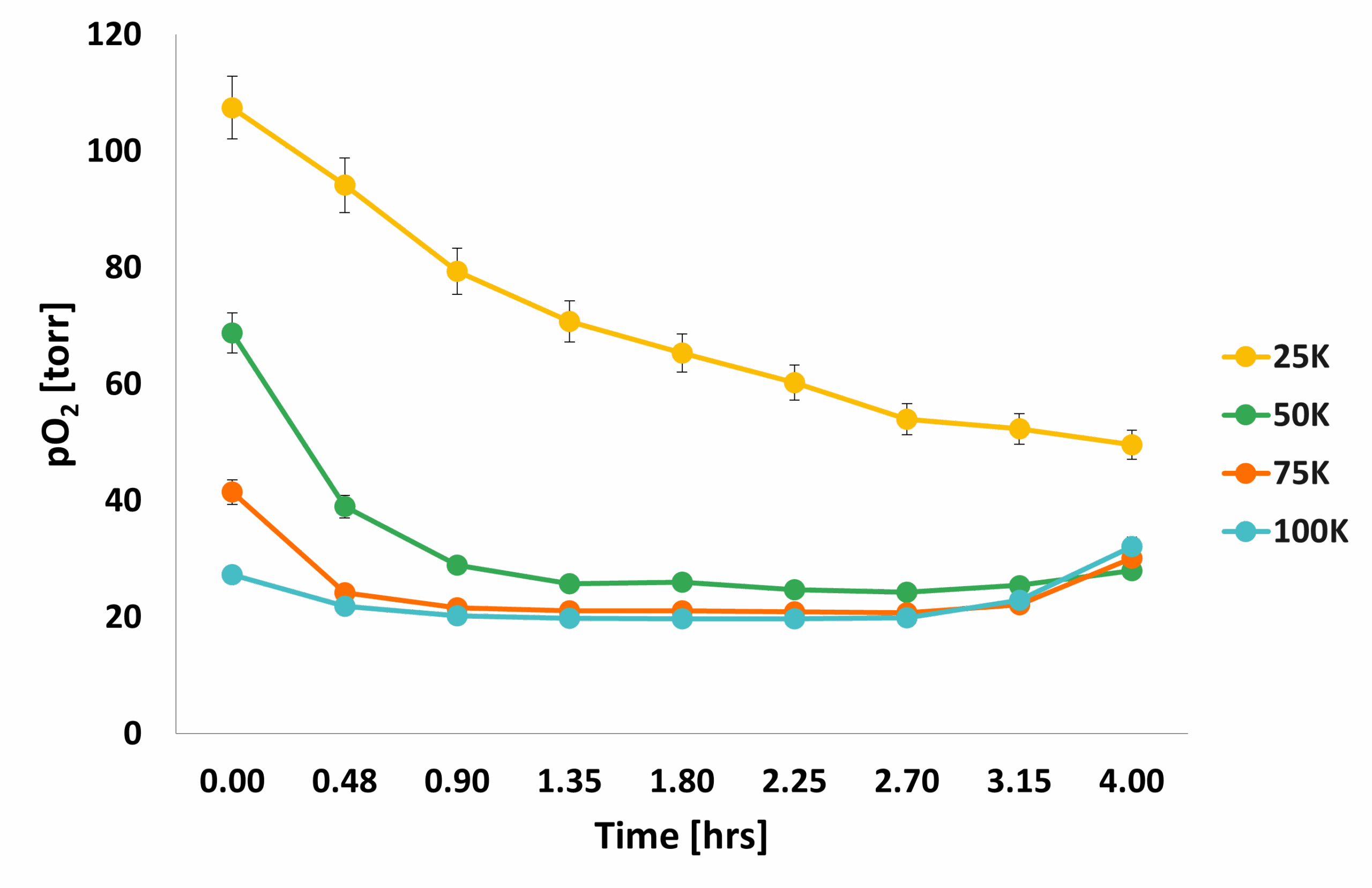
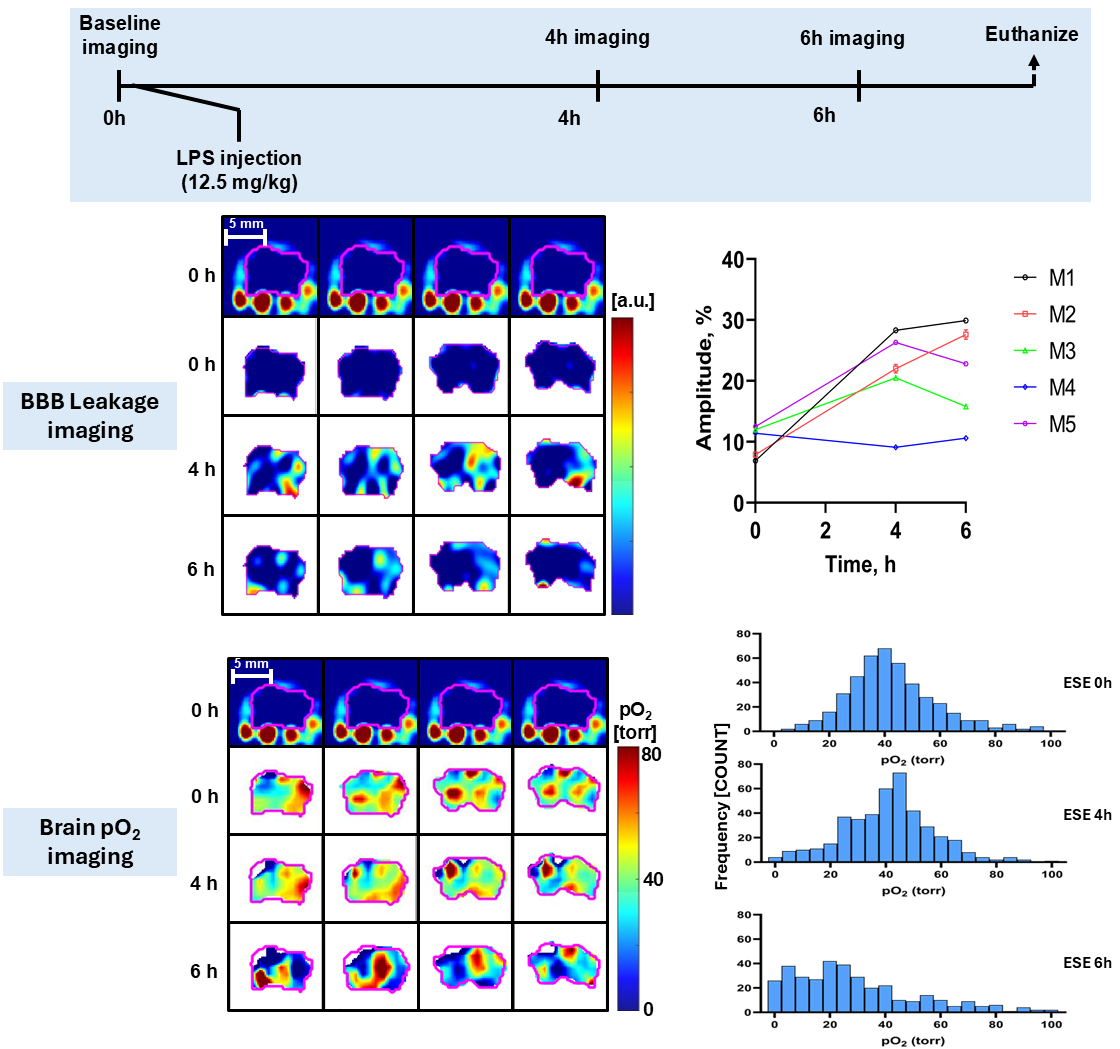
The following figure shows how the average pO2 evolved over the course of 4 hours: the mean pO2 of 50K cells matched the pO2 of wells with 100K cells, where cell dormancy or cell death was probably happening because of overcrowding. The pO2 for 25 K cells dropped over time, indicating increased metabolic activity in these wells.
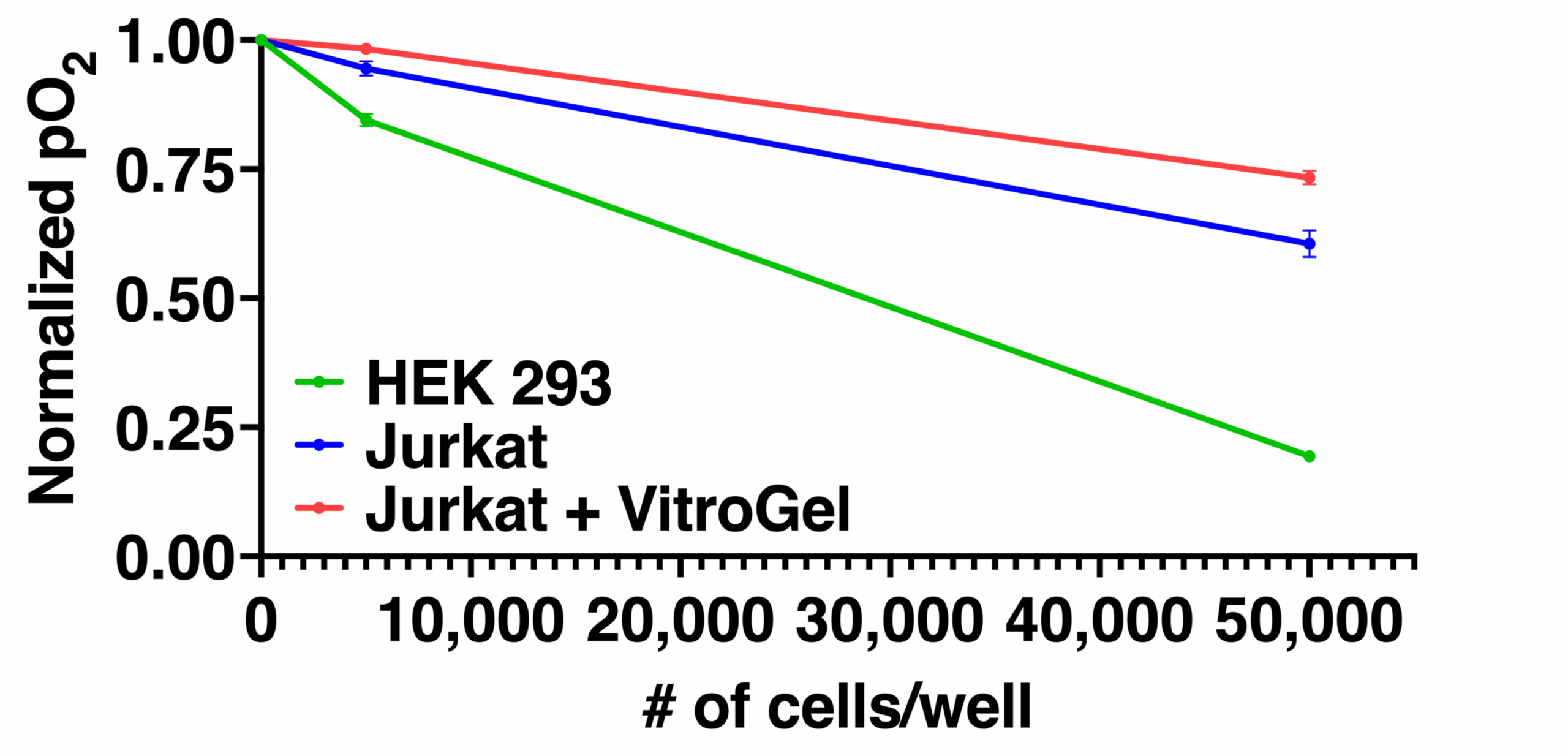
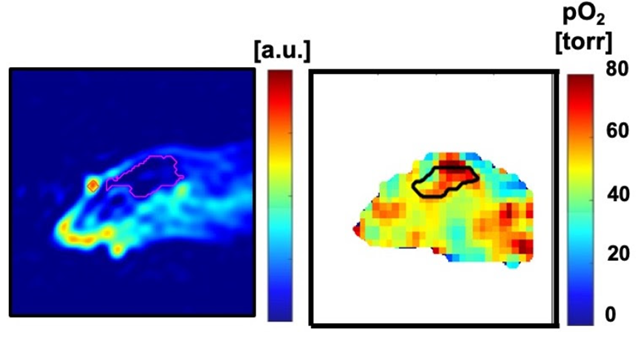
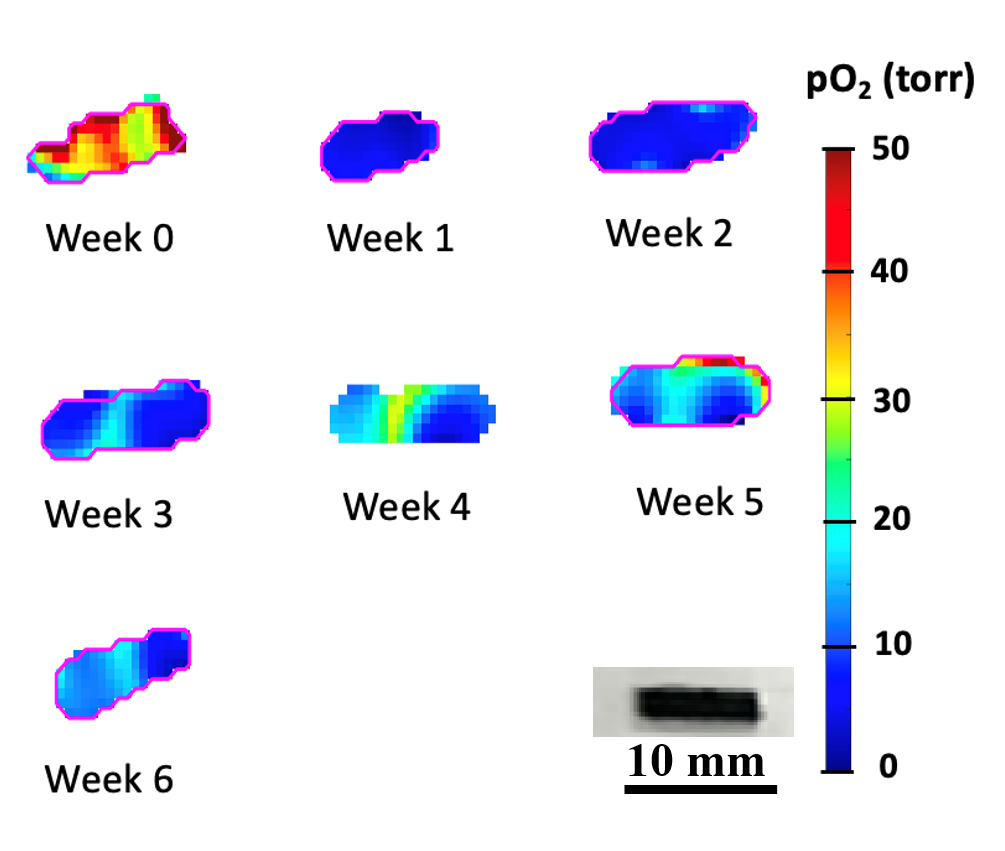
An important challenge in evaluating tissue-engineered medical products arises when cells are implanted into a hydrogel scaffold because there is no method available to monitor their metabolic activity without disrupting the structure. In the figure below, we show that the quantitative estimate of cell loss for cells seeded in a biomaterial can be obtained using oxygen imaging.
In the Figure below, we demonstrate the 3D cell viability assessment using oxygen imaging.
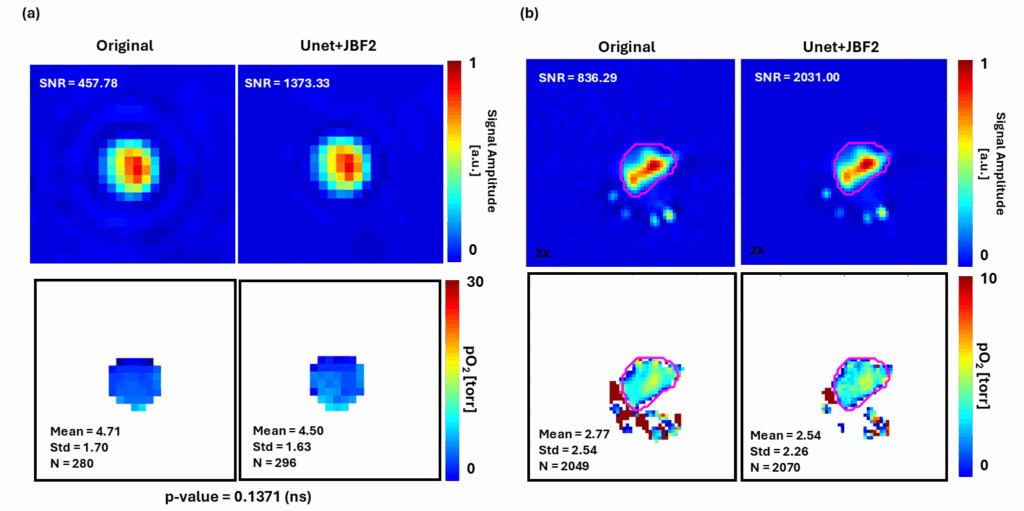
This is the first study showing nondestructive, 3D, longitudinal cell viability assessment using 3D oxygen imaging and it may impact cell therapy, tissue engineering, regenerative medicine, and other fields. All experiments were performed using O2M's preclinical oxygen imager, JIVA-25®.
This is an important assay for every biological lab, as the cells subjected to different oxygen concentrations can lead to vastly different outcomes!
Click here to access the full paper and learn more!
Oxygen Imaging of Cells for Viability Assessment Read More »