Type 1 diabetes (T1D) is an autoimmune disease that leads to the loss of insulin-producing pancreatic beta cells. Beta cell replacement devices or bioartificial pancreas (BAP) have shown promise in curing T1D and providing long-term insulin independence without the need for immunosuppressants. However, hypoxia in BAP devices can damage the cells and limit device dimensions. Noninvasive in vivo oxygen imaging assessment of implanted BAP devices will provide the necessary early feedback and improve the chances of success. In the study titled “In Vivo Mouse Abdominal Oxygen Imaging and Assessment of Subcutaneously Implanted Beta Cell Replacement Devices“, published in Molecular Imaging and Biology journal, we optimized mouse abdominal oxygen imaging using our core technique, electron paramagnetic resonance oxygen imaging (EPROI) and our novel oxygen imager, JIVA-25®, to assess BAP devices in vivo. This study is part II of our previously published study demonstrating in vitro oxygen assessment of BAP devices. 
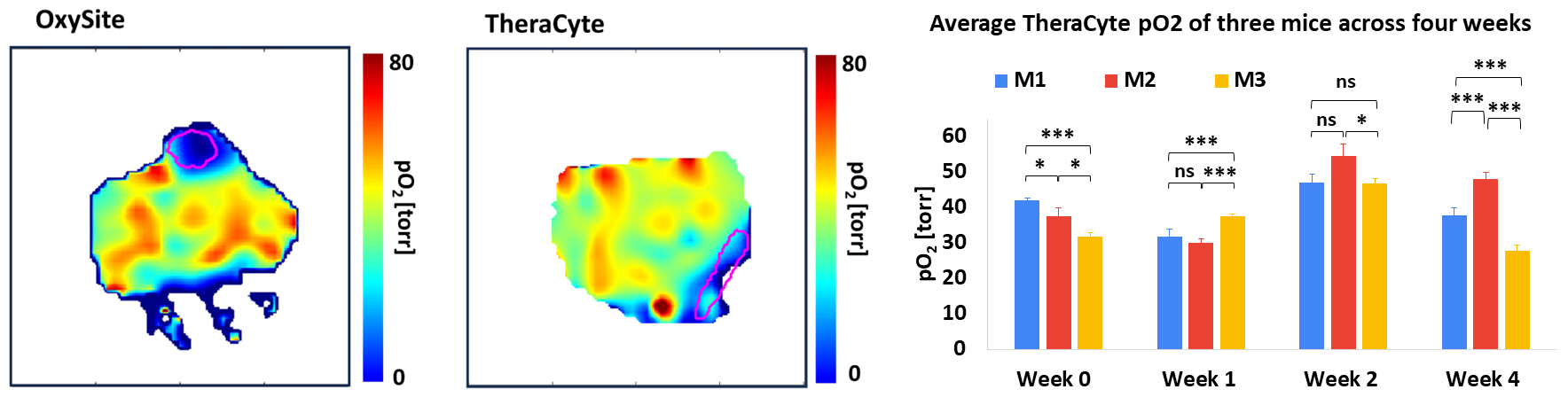
We demonstrated proof-of-concept pO2 imaging of two subcutaneously implanted BAP devices: an agarose-based cylindrical device (OxySite) implanted in streptozotocin (STZ)-induced diabetic animals and a commercially available cell encapsulation device (TheraCyte) implanted in non-diabetic animals. Check out the full study here.