Cancer is a major public health problem worldwide and is the second leading cause of death in the United States. About two-thirds of cancer patients are treated with radiation, either alone or in combination with immunotherapy, chemotherapy, or surgery. It is known that hypoxia or low partial oxygen pressure (pO2) causes resistance to radiation therapy, immunotherapy, and chemotherapy.
Solid tumors treated with radiotherapy often experience decreased oxygen delivery. This early tumor response to radiotherapy can be monitored by measuring the pO2 in the tumor microenvironment. These in vivo pO2 measurements can be accomplished using the JIVA-25™ Electron Paramagnetic Resonance Oxygen Imaging (EPROI) instrument from O2M Technologies. During EPROI studies, a gas anesthetic is often used to immobilize the mouse. Unfortunately, a gas anesthetic that uses medical grade air (21% O2) or 100% O2 breathing gas can alter the measured pO2 in the tumor, potentially complicating the interpretation of the imaging results.
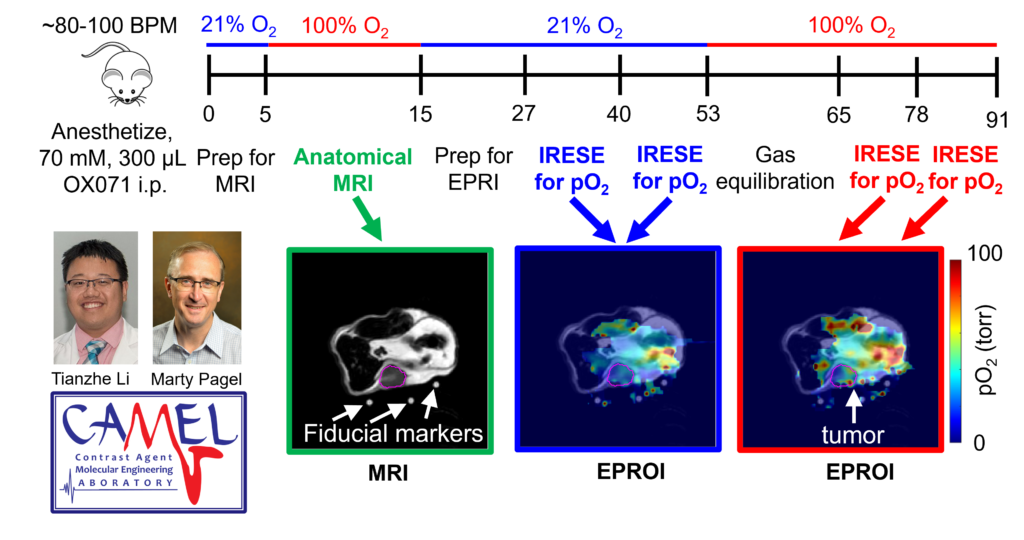
Figure 1. Oxygen Enhanced EPROI. The workflow acquires anatomical MR images, two pO2 maps with 21% O2 breathing gas, and two pO2 maps of 100% O2 breathing gas. The test-retest shows outstanding precision of 3.1 Torr with both breathing gases.
Prof. Marty Pagel and graduate student Tianzhe Li at the MD Anderson Cancer Center have exploited this potential pitfall to develop a new biomarker for assessing early tumor response to radiotherapy. They have measured ΔpO2, which is the difference in pO2 measured with 21% O2 and 100% O2 breathing gases. As an analogy, a car with a ¼-full gas tank has an available capacity of ¾-empty tank. ΔpO2 represents the available capacity of a tumor to take more O2. To demonstrate the value of this new biomarker, Li and Pagel have developed an Oxygen Enhanced (OE)-EPROI protocol that interperitoneally administers OX071 agent, acquires MR images for anatomical reference, and then obtains pO2 maps with 21% O2 and 100% O2 breathing gases (Figure 1). They have applied their protocol to study the early response of a Colo357 model treated with 10 Gy radiotherapy (Figure 2).
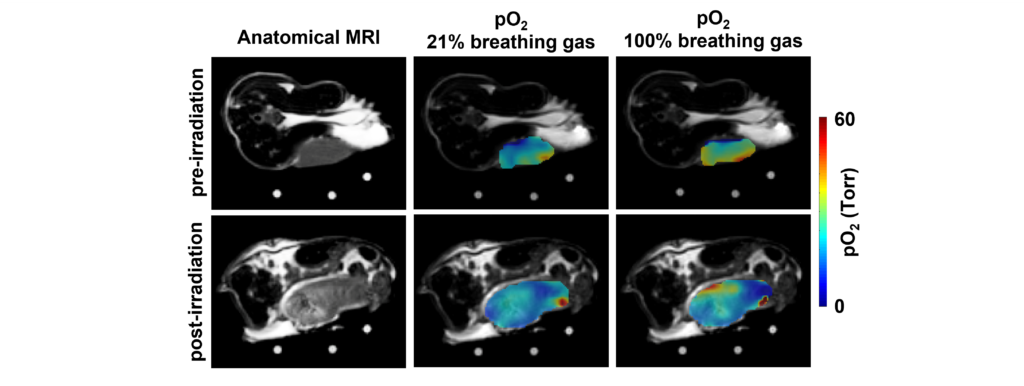
Figure 2. Oxygen Enhanced (OE)-EPROI showing a significant decrease in ΔpO2.
Their results have shown that radiotherapy caused a large and significant decrease in ΔpO2 (Table 1). For comparison, radiotherapy caused a small, statistically insignificant decrease in tumor pO2 with 21% O2 breathing gas. Similarly, radiotherapy caused a larger yet still statistically insignificant decrease in tumor pO2 with 100% O2 breathing gas. Furthermore, ΔpO2 showed the greatest effect size, known as the change in a biomarker relative to its variance, demonstrating that ΔpO2 was most effective in evaluating an early tumor response to radiotherapy. This OE-EPROI protocol can provide a new diagnostic method for evaluating early response to radiotherapy.
Table 1. OE-EPROI of the Colo357 model undergoing radiotherapy (n=9)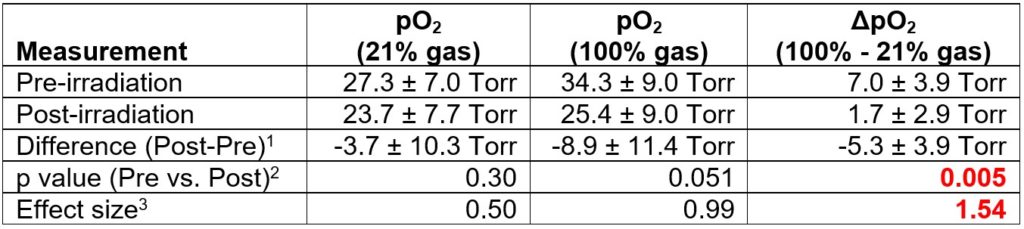
- differences determined for each mouse, and not based on the averages of the groups.
- p < 0.01 is considered to be statistically significant, from a two-tailed Student’s T-Test assuming equal variances
- Effect size: |{(average post-pre) / (average standard deviation of post and pre)}|
Prof. Marty Pagel leads the Contrast Agent Molecular Engineering Laboratory (CAMEL) at the MD Anderson Cancer Center, Houston TX, USA. CAMEL evaluates hypoxia, acidosis, enzyme activity and vascular perfusion in the tumor microenvironment, using a variety of molecular imaging methods. More specifically, CAMEL uses EPROI to evaluate chemotherapy, immunotherapy, radiotherapy and radiosensitizers in small animal models of cancer and models of wound healing.
